Perioperative analgesic pharmacology in children

This page is under construction, converting the originally formatted pdf from the WFSA site with wiki embellishments.
Originally from Update in Anaesthesia | www.wfsahq.org/resources/update-in-anaesthesia
Reprinted with revisions from: Williams G. Perioperative analgesic pharmacology in children. Anaesthesia Tutorial of the Week 23 (2006)
Glynn Williams Consultant Anaesthetist Great Ormond Street Hospital for Children NHS Trust UK. Correspondence email: willig3@gosh.nhs.uk
INTRODUCTION
The inadequate treatment of pain in children following surgery was first highlighted over 20 years ago. A survey at the time found that 40% of paediatric surgical patients experienced moderate or severe postoperative pain and that 75% had insufficient analgesia. Since then increased interest in this area has led to a better understanding of the developmental neurobiology of pain and analgesic pharmacology and, consequently, allowed for the development of safer and more effective analgesic techniques for children of all ages.
PAIN PERCEPTION
During foetal, neonatal and infant life the nervous system is continually evolving. This allows structural and functional changes to occur continuously in response to the child`s needs as it grows and develops. The pain pathways mirror these changes with different components developing along differing time frames. For instance the structural components required to perceive pain are present from early foetal life whereas pathways involved in modifying pain perception are still developing during infancy. Also opioid and other receptors vary in their number, type and distribution between early life and adulthood.
| Summary |
|---|
| It is both desirable and possible to achieve safe, effective analgesia for children of all ages using individualised pain management planning and combined multimodal analgesic techniques |
The challenge of treating pain in these young age groups is to understand how this changing nervous system affects the child’s perception of pain and the efficacy and safety of analgesic treatments. The field is the subject of much research but there are many gaps in our knowledge that need to be filled. Pain is a subjective experience and is thus difficult to assess if communication is not possible. Assessment relies on using non-specific behavioural and hormonal signs of distress/stress. It has been shown in neonates and infants that the use of adequate perioperative analgesia will modify behavioural and hormonal stress responses and reduce morbidity.
PERIOPERATIVE PAIN MANAGEMENT
Successful pain management is based on the formulation of a sensible analgesic plan for each individual patient. It is best to take a practical and pragmatic approach dependent on the patient, the type of surgery and the resources available. Realistic aims are to recognise pain in children, to minimise moderate and severe pain safely in all children, to prevent pain where it is predictable, to bring pain rapidly under control, and to continue pain control after discharge from hospital.
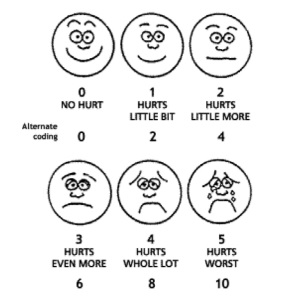
Once a pain management plan is implemented it should be regularly reassessed and changes made as required. Appropriate pain assessment is vital to aid this. This should involve clinical assessment of the child and the use of an appropriate pain scoring tool to identify discomfort and monitor the efficacy of any analgesic intervention. Due to the subjective nature of pain and the lack of a reliable measure many different tools are available. If the child is able to communicate their pain then a self reporting score, such as the “pain faces” (see figure 1) should be used. If the child cannot communicate then other tools using behavioural and physiological signs should are appropriate, the one chosen from the many available will depend on the age of the child, their neurological and cognitive state, and local preference.
The plan should also include provision for the rapid control of pain that is not alleviated by the original treatment (breakthrough pain) and the identification and treatment of side-effects. In established paediatric centres with high level of resources a dedicated paediatric pain service is the standard of care. Where this is not available significant improvements in pain management can be made by the establishment of clinical routines and protocols for the treatment and assessment of postoperative pain and a network of interested medical and nursing staff to provide ongoing education.
MULTIMODAL ANALGESIA
Multimodal, or balanced, analgesia, involves the simultaneous use of a number of analgesic interventions to achieve optimal pain management. Our increased understanding of pain biology has allowed us to use analgesic techniques that modify nociceptive transmission at different points along the pain pathway. This produces analgesia using minimal doses of drugs, thereby reducing side-effects.
Using a multimodal approach effective pain management is achievable for most cases and the technique can be adapted for day cases, major cases, the critically ill child, or the very young. In current practice most analgesic techniques are based on differing combinations of four main classes of analgesics: paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs), opioids, and local anaesthetics. Though it is not uncommon for other agents, e.g. NMDA antagonists and alpha-2 agonists, and non pharmacological analgesic methods to be used in addition. Unless there is a contraindication to do so, a local/regional analgesic technique should be used in all cases. For many minor and day case procedures this, in combination with paracetamol and NSAIDs, may allow opioids to be omitted.
Paracetamol (Acetaminophen)
Paracetamol has a mainly central mode of action producing both antipyretic and analgesic effects. It has been shown to inhibit prostaglandin synthesis in the hypothalamus, reduce hyperalgesia mediated by substance P and reduce nitric oxide generation involved in spinal hyperalgesia induced by substance P or NMDA.
It is the most widely prescribed drug in paediatric hospitals and has become the mainstay base analgesic for almost all procedures. The analgesic potency is relatively low and on its own it is only really effective against mild pain. In combination, however, with NSAIDs and weak opioids it has been shown to be effective for moderate pain and it has also been shown to have an opioid sparing effect when used in tandem with the more potent opioids.
The oral bioavailablity of paracetamol is very good as it is rapidly absorbed from the small bowel. Rectal absorption is slow and incomplete, except in neonates. Though the formulations of different brands of suppository vary and the more lipophillic the better the bioavailability. Even so, if paracetamol is given rectally at the start of a short procedure (<1hour) it is unlikely to reach therapeutic plasma levels by the time the child wakes in the recovery room. Thus, if possible, paracetamol should be given orally and pre-operatively. Intravenous paracetamol preparations are becoming widely available in many countries. Studies suggest that it may have a higher analgesic potency than either the oral or rectal preparations though the time to peak analgesia is still 1–2 hours. Irrespective of the route of administration a regular rather than an “as required” post-operative prescription has been shown to provide better analgesia.
In recent years the accumulation of a large body of evidence concerning the use of paracetamol in children has led to the revision of dosing schedules to ensure a balance between efficacy and safety. Smaller doses and longer dosage intervals are required in the neonate or sick child.
Non-steroidal anti-inflammatory drugs (NSAIDs)
NSAIDs act mainly peripherally by inhibiting prostaglandin synthesis and thus reducing inflammation, although central effects have also been postulated involving the opioid, serotonin and nitric oxide pathways. They are highly efficacious in their own right in the treatment of mild to moderate pain in children. They have a reported
| Age | Oral: Loading dose | Maintenance dose | Rectal: Loading dose | Maintenance dose | Maximum daily dose | Duration at maximum dose |
|---|---|---|---|---|---|---|
| Pre term <32 weeks | 20mg.kg-1 | 15mg.kg-1 up to 12 hourly | 20mg.kg-1 | 15mg.kg-1 up to 12 hourly | 45mg.kg-1.day-1 | 48h |
| 32 weeks – 1 month | 20mg.kg-1 | 15mg.kg-1 up to 6 hourly | 30mg.kg-1 | 15mg.kg-1 up to 6 hourly | 60mg.kg-1.day-1 | 48h |
| >1month | 20mg.kg-1 | 15mg.kg-1 up to 4 hourly | 30-40mg.kg-1 | 20mg.kg-1up to 6 hourly | 90mg.kg-1.day-1 | 72h |
| Age/Weight | Dose | Maximum daily dose |
|---|---|---|
| 1month – 50kg | 15 mg.kg-1 up to 6 hourly | 60 mg.kg-1/day |
| >50kg | 1g up to 6 hourly | 4 mg.kg-1 |
| Drug | Route | Dose |
|---|---|---|
| Ibuprofen | Oral | <20kg: 5-10mg.kg-1 up to 6 hourly
>20kg: 200mg 6 hourly |
| Diclofenac | Oral/Rectal | 1-3mg.kg-1 up to 3mg.kg-1 in divided doses |
opioid-sparing effect of 30 – 40 % when used in combination with opioids and also reduce opioid-related adverse affects as well as facilitating more rapid weaning of opioid infusions. They have also been shown to be highly effective in combination with local or regional nerve block. Combination with paracetamol produces better analgesia than either drug alone.
There are limitations to their use in paediatric populations. At present, in general, their use is not recommended for children less than 6 months of age, although in the UK Ibuprofen is now available over the counter in formulations for ages 3 months and above. Care should also be taken in those patients who are asthmatic, have a known aspirin or NSAID allergy, are hypovolaemic or dehydrated, renally-impaired, coagulopathic or where there is a significant risk of haemorrhage. A careful history of previous use of NSAIDs should be taken in every case. Different NSAIDs have different side-effect profiles and the relative risks associated with each of the contraindications given will differ between drugs. In the UK the Committee on the Safety of Medicines has classified Ibuprofen and Diclofenac as having the best side-effects profile.
Pharmacokinetic studies have indicated a higher than expected dose requirement in children if scaled by body weight from adult doses. Rectal and oral bioavailability are both good though again for short cases they are best give orally preoperatively. Other routes of administration are available, such as intravenous and topical, though their use in children has been limited so far.
Opioids
As with adults, opioids, and morphine in particular, remain the mainstay of analgesic treatment for the majority of all but minor surgical procedures. The choice of which opioid to use will depend on the patient`s medical history, the type of surgery, drug availability, any locally devised protocols and, often, individual anaesthetic preference. The pharmacology of these agents changes during early life and these changes are not consistent between different drugs. When using a particular opioid in neonates and infants it is important to understand the pharmacology of that particular drug in those age groups to ensure both efficacy and safety.
Morphine remains the most commonly used opioid and, consequently, is the most studied. Morphine clearance is decreased and the elimination half-life is increased in neonates compared with infants and older children. Also in neonates the glucuronidation pathways, the main metabolic pathway for morphine, are still developing, slowing morphine metabolism and giving a relatively increased production of morphine-6-glucuronide, an active metabolite of morphine. These differences may to some extent account for the increased efficacy of morphine seen clinically in neonates. Codeine however, another popular opioid in neonates and infants, works via metabolism to morphine. The cytochrome P450 enzyme responsible for this conversion shows markedly reduced activity at these ages compared with that seen in older children and adults. Thus it may be that little or no morphine is produced from a dose of codeine. This may explain codeine`s good safety profile in young children but may also suggest that the analgesic efficacy is questionable. Recently there has been increased awareness of children suffering respiratory depression following codeine, this is thought to be due to ultra-fast metabolism converting codeine into its active metabolite, morphine. In UK it is no longer recommended to use codeine in any child under 12 years old for this reasons. It is not possible to predict who will metabolise codeine quickly or poorly.
Many routes for the administration of opioids are available in children. During surgery, with an IV cannula in situ, the intravenous route is the easiest. In terms of bioavailability and consistency of effect it is also the most reliable. For these reasons it is usually the route of choice postoperatively, especially after major surgery. Safety, however, must always be a priority. Potentially serious complications such as over-sedation and respiratory depression can occur even when using well constructed protocols for opioid use. With infusion or bolus techniques safe practice must include the presence of appropriately educated staff and regular observation of sedation and respiratory rate. Oxygen and opioid antagonists should be easily accessible in case of emergency. If these are not available then other routes of administration or analgesic strategies are indicated. It is sensible for each institution to devise protocols for opioid use dependent on their own local resources.
Oral and rectal formulations of many opioids are available and have a place for some patients in the perioperative period, especially if safety is a prohibitive local issue for other methods of delivery. If the IV route is available postoperatively, continuous IV infusions and intermittent intravenous boluses have been shown to be safe and effective. Where local resources allow, IV opioids are usually administered via patient (PCA) or nurse (NCA) controlled delivery systems which demonstrate good efficacy and safety in all age groups, even neonates. The subcutaneous route remains an alternative to IV administration but absorption is not as reliable and it should not be used in hypovolaemic patients. Intramuscular injection demonstrates slow absorption and unreliable effect and is considered undesirable in the awake child due to the pain and distress of the injection. Other routes of administration are available, e.g. sublingual, transdermal and intranasal and may be appropriate in specific cases or where local resources dictate their use. Opioids are also commonly used as adjuncts to local anaesthetics.
| Titrated loading dose of IV morphine
50mcg.kg-1 increments, repeated up to x4 |
| Ongoing bolus dose of IV morphine
20mcg.kg-1 per bolus, repeated to effect |
| Oral morphine*
80mcg.kg-1 4hrly (1month – 1yr) 200 – 400mcg.kg-1 4 hourly (>1yr) |
| IV or s.c. morphine infusion
10 – 40mcg.kg-1.hr-1 |
| PCA with morphine
Bolus dose 10 – 20mcg.kg-1 Lockout interval 5min Background infusion 0 – 4mcg.kg-1.hr-1 |
| NCA with morphine
Bolus dose 10 – 20mcg.kg-1 Lockout interval 20 - 30min Background infusion 4 – 20mcg.kg-1.hr-1** |
| * Can be used in neonates at 80mcg/kg but may need to increase dosage interval to 6 hourly and child must be closely monitored.
**For neonates a background dose is omitted to reduce the possibility of a prolonged effect of the morphine. This allows the carer to use the bolus function to titrate the analgesia and anticipate painful episodes. |
Local Anaesthetics (see chapters on specific local anaesthetic blocks)
Local anaesthetic agents work by blocking the conduction of nociceptive stimuli along the pain pathway. This can be achieved by many different routes, the commonest being central/regional blocks, plexus blocks, peripheral nerve blocks, infiltration at the site of injury, and topical application. Some method of nerve blockade is appropriate for nearly all surgical procedures and forms an important part of a balanced analgesic technique. Though, once again, the technique used will depend on the patient’s medical history, the type of surgery, any locally devised protocols and, often, individual anaesthetic preference.
Regional analgesia produces excellent perioperative analgesia for major surgery at all age groups, even preterm neonates, and has been shown to decrease postoperative complications. The evidence for this, however, is limited as is the evidence for the risks associated with paediatric epidural analgesia. In general these techniques should only be performed by experienced practioners with appropriately trained staff and protocols and monitoring guidelines available for the postoperative period. Plexus or peripheral nerve blocks have been shown to have good efficacy and safety for limb and head and neck surgery. In some procedures involving the lower limbs the analgesia obtained from a peripheral nerve block has a much longer duration of action than that obtained from a single shot central block.
For many years bupivacaine has been the local anaesthetic of choice in paediatric practice. It has been extensively studied and safe-dosing guidelines have been established which have greatly reduced the incidence of systemic toxicity. Neonates demonstrate decreased clearance and decreased protein binding of local anaesthetic agents. Therefore, at this age, there is a risk of systemic toxicity and dosing schedules have to be adjusted. Although thankfully rare, bupivacaine still carries the risk of significant cardiotoxicity and this has lead to the increasing introduction of the newer agents, ropivacaine and levo-bupivacaine (chirocaine), into current practice. There are now sufficient paediatric data to recommend either of these agents. Adjuncts such as opioids, adrenaline, ketamine and clonidine are commonly added to local anaesthetic agents. This is mainly seen with regional/ central blockade. They allow for increased duration and spread of the block with minimal increase in side-effects, due to the low doses used.
DISCHARGE FROM HOSPITAL
A sensible analgesic plan should include provision for analgesia to continue once the child has been discharged from hospital. Clear and easy to follow instructions should be given to aid compliance and thus efficacy. Inadequate pain relief at home, as in hospital, will lead to unacceptable distress for both child and carer and the potential for complications. In general an assessment is needed of the likely severity and duration of the pain. Again a multi-modal approach should be adopted and regular dosing schedules are superior to “as required” prescriptions when the pain is likely to be significant.
If a single bolus local anaesthetic technique has been used then the time that this will wear off must be anticipated and analgesic provision made. Often analgesia is required for a significant period of time after discharge. For instance it is well described that children still experience significant pain 7-10 days following adenotonsillectomy.
Table 4. Dosing guide for bupivacaine, levobupivacaine and ropivacaine:
| Single bolus injection
Neonates Children |
Maximum dosage
2mg.kg-1 2.5mg.kg-1 |
| Continuous Infusion
Neonates Children |
Maximum infusion rate
0.25mg.kg-1.hr-1 0.5mg.kg-1.hr-1 |
SUGGESTED READING
[http://www.wfsahq.org/components/ com_virtual_library/media/e558e631b7d38a387f407ae65805690c490d426becffb3927dcc16bd60ef9805-23-perioperative-analgesicpharmacology-for-children.pdf Williams G. Perioperative analgesic pharmacology in children. ATOTW (2006) 23.]
1. Lonnqvist P-A, Morton NS. Postoperative analgesia in infants and children. Br J Anaesth 2005; 95: 59 – 68.
2. Berde CB, Sethna NF. Analgesics for the Treatment of Pain in Children. N Engl J Med 2002; 347: 1094 – 103.
3. Howard RF, Lloyd-Thomas A. Pain management in children. In: Sumner E and Hatch DJ eds. Paediatric Anaesthesia. London, U.K. Arnold. 1999.
4. [http://onlinelibrary.wiley.com/doi/10.1111/pan.2012.22.issue-s1/ issuetoc#group1. Good Practice in Postoperative and Procedural Pain Management, 2nd Edition, 2012 (79 page special issue, free to download).]
5. [http://www.apagbi.org. uk/sites/default/files/images/Codeine_Nov2013.pdf. Association of Paediatric Anaesthetists & Royal College of Paediatrics and Child Health. Guidance for the administration of codeine and alternative opioid analgesics in children. 2013.]