Basic Science Relevant to Practical Pediatric Anesthesia: Difference between revisions
m 1 revision imported |
|
(No difference)
| |
Revision as of 02:34, 5 March 2021

This page is under construction, converting the originally formatted pdf from the WFSA site with wiki embellishments.
Originally from Update in Anaesthesia | www.wfsahq.org/resources/update-in-anaesthesia by Anthony Short* and Kate Stevens (*Correspondence Email: tone_short@hotmail.com)
Anthony Short:
- ST7 Anaesthetics,
- Morriston Hospital,
- Swansea,
- South Wales
Kate Stevens:
- Consultant Anaesthetist and Intensivist
- Morriston Hospital,
- Swansea,
- South Wales
It is often said that paediatric patients are ‘not simply small adults’. The truth is that from the premature neonate to the near-adult adolescent, children are very diverse (see Table 1 for age definitions used in this article). This article will consider the basic science and calculations commonly used in paediatric anaesthesia; our challenge is to consider the anatomical, physiological and other differences that impact on anaesthetic practice.
Estimation of weight
It is essential that every child is weighed prior to anaesthesia. This allows correct calculation of drug doses and selection of anaesthetic equipment. In emergencies, weight can also be estimated from the age of the child from growth charts (use the weight at the 50th centile), from the length of the child using a Broselow Tape, or using the formulae shown here:
| Age of child | Formula to estimate weight in kg |
| 0-12 months | (0.5 x age in months) +4 |
| 1-5 years | (2x age in years) +8 |
| 6-12 years | (3x age in years) +7 |
Airway and respiratory tract
Anatomical differences of the paediatric airway influence airway management and the selection of appropriate equipment. The major anatomical differences affecting airway management in neonates and infants are as follows:
- •Relatively large head and prominent occiput
- •Small mandible
- •Relatively large tongue
- •Short neck
- •Soft tracheal cartilages, easily compressed.
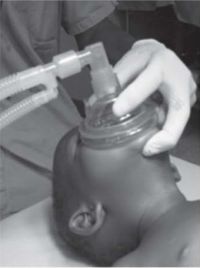
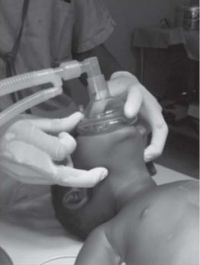
These differences predispose to airway obstruction, particularly if the child’s head is placed on a pillow, or the soft tissues on the floor of the mouth are compressed, or the head is hyperextended. Ideally, maintain the child’s head in a neutral position, or slightly extended, possibly with a small pad under the shoulders, and open the airway using a chin lift or jaw thrust, avoiding compression of the soft tissues of the floor of the mouth (see Figure 1).
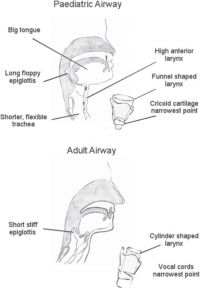
Anatomical differences affecting the larynx include:
- •A high, anterior position of the larynx (level of C3-4 in infants compared to C5-6 in adults)
- •A long, U-shaped epiglottis projecting at 45 degrees posteriorly
- •A ‘funnel shaped’ larynx. The narrowest part of the airway is at the cricoid cartilage (below the vocal cords). The narrowest part of the airway in adults is at the vocal cords. (More recent literature suggest that this is not the case, and the narrowest part is still the cords, even in children. See...Pediatric airway management Jeff Harless, Ramesh Ramaiah, and Sanjay M Bhananker)
- •A thin loose lining to the airway which is easily damaged
The intubation technique in infants needs to take account of these anatomical differences:
- •Prepare all your equipment, find an assistant, monitor the child and preoxygenate; give yourself enough time
- •Handle the tissues gently and choose the appropriate sized tube. Multiple attempts at intubation may result in postextubation stridor
- •Place the head in a neutral position or slightly extended, and stabilise the head with your right hand; use your right index finder to open the mouth
- •Hold the laryngoscope close to the hinge of the blade, using the thumb and index finger of your left han.
- •If necessary, use the little finger of your left hand to press on the larynx to bring the laryngeal structures into view.
- •Place the tip of the larygngoscope blade into the vallecula, the space above the epiglottis (curved blade), or beneath the epiglottis (straight blade) to lift the epiglottis to expose the larynx and vocal cord.
- •Pass the tube carefully between the vocal cords, under direct vision. Do not insert the tube too far; the tracheal length is approximately 4.5cm in most infants.
Adenotonsillar hypertrophy is common in children 3 – 8 years of age. Airway obstruction may develop when pharyngeal tone is lost after induction of anaesthesia; an oropharyngeal may help to maintain a patent airway. Take care when passing nasopharyngeal, nasotracheal and nasogastric tubes in these children. Children aged 5-13 years may have loose teeth; take note of loose teeth at your preassessment visit.
Respiratory considerations
Up to 6 months of age, infants are almost exclusively breast fed, and need to breathe through their nose rather than their mouth (obligate nasal breathers). Respiratory difficulties may result if the nose is blocked, for instance due to secretions from upper respiratory tract infections, or if a nasogastric tube is present.
Neonates have very limited respiratory reserve, and become hypoxic very easily. They have a high metabolic rate and twice the oxygen consumption compared to older children and adults (6-7ml.kg-1.min-1 in neonates compared to 3-4 ml.kg-1.min-1 in adults).
The respiratory exchange surface is immature, with only 1/10 the number of alveoli compared to adults; in premature neonates this is compounded by a lack of respiratory surfactant that helps to reduce surface tension and to stabilise the alveolar air spaces. The lack of surfactant in premature infants predisposes them to airway collapse, poor gas exchange and increased work of breathing. Ventilation with high airway pressures and high-inspired oxygen concentration predisposes to bronchopulmonary dysplasia and chronic lung disease. The airways are very small in neonates, and easily obstructed. The flow in the airway can be described by the Hagen Pouiselle formula, assuming laminar flow:
- Q =(∆P π r4) / (8 µ L)
where
- Q = volumetric flow rate
- ∆P = pressure drop
- π = a constant
- r = radius
- µ - dynamic viscosity
- L – airway length
The flow is therefore proportional to the radius^^4; halving the
radius of the airway decreases the flow rate by a factor of 16.
A small amount of airway oedema from a difficult intubation,
or infection, or respiratory secretions, will significantly reduce
airflow and increase the work of breathing for a neonate.
Respiratory mechanics in the neonates are not very efficient.
The rib cage is soft and compliant, and the ribs move in
the horizontal plane only (rather than in the horizontal and
anterior-posterior direction in adults, like a bucket handle).
This means the tidal volume is relatively fixed (5-7 ml.kg-1), and
the infant can only increase minute ventilation by increasing
respiratory rate (see Table 3 for normal values). Deadspace
volumes should be kept to a minimum for neonates and infants
to reduce the work of breathing and to reduce re-breathing.
The diaphragm is the main muscle of respiration in neonates
and infants, but is prone to fatigue due to a lack of type 1
(oxidative, fatigue resistant) muscle fibres. The diaphragm
may be splinted by gastric distension due to swallowed air (very common with crying or facemask ventilation), or due
to bowel obstruction. It is important to consider placement of
a nasogastric tube to decompress the stomach in infants with
abdominal distension or respiratory distress.
The soft rib cage means that the chest wall in babies is highly
compliant, and there is less ‘outward spring’ exerted by the
rib cage, and less negative intrapleural pressure to keep the
lungs expanded. This results in a relatively low functional
residual capacity (FRC), and airway closure may occur during
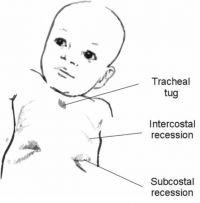
normal breathing. Intercostal and sternal recession is common
in babies if there is any airway obstruction, or reduction in
lung compliance (for example due to infection) (see figure 3).
Tracheal tug also occurs. An infant with respiratory distress
may ‘grunt’ to maintain airway volumes – there is partial
closure (adduction) of the vocal cords during expiration,
which effectively provides physiological continuous positive
airway pressure (CPAP), and helps to keep the airways open.
Conversely, the presence of recession, tracheal tug and grunting
in an older child with a more rigid rib cage is an ominous
sign, and suggests very severe respiratory distress. Increased
respiratory rate is an important sign of respiratory distress at
any age.
| Age (years) | Respiratory rate (breaths per minute) Rate reduces with increasing age | Heart rate (beats per minute) Rate reduces with increasing age | Blood pressure (mmHg) Pressure increases with increasing age |
| <1 | 40-30 | 160-110 | 80-90 |
| >1-2 | 35-25 | 150-100 | 85-95 |
| >2-5 | 30-25 | 140-95 | 85-100 |
| >5-12 | 25-20 | 120-80 | 90-110 |
| >12 | 20-15 | 100-60 | 100-120 |
Table 3. Cardiorespiratory physiology – normal values
The control of respiration is immature in neonates. Responses
to hypercarbia and hypoxia are blunted and poorly sustained,
and neonates often respond to hypoxia by stopping breathing
(apnoea).
Apnoeas are particularly common in premature and ex-premature infants, and are significant if they last longer than 15 seconds or are associated with desaturation or bradycardia. Volatile anaesthetics and opioids reduce the respiratory drive further, and should be used cautiously in neonates, especially premature neonates. Consider opioid sparing techniques (paracetamol, local anaesthetic blocks) whenever possible. Caffeine given orally prior to surgery has been used to reduce the risk of apnoea. All ex-premature neonates <60 weeks post conception should be monitored carefully after an anaesthetic, ideally with an apnoea monitor if available. Term neonates are also susceptible to apnoeas after routine anaesthesia for minor procedures, likely until they are at least a month old. Premature infants (before 35 weeks gestational age) are at risk of retinopathy of prematurity due to abnormal vessel proliferation in the vitreous of the eye, which may result in haemorrhage, scarring and retinal detachment, and is a common cause of blindness in this population. High PaO2 may worsen retinopathy, which is also seen in term infants given unmonitored oxygen therapy. If oxygen therapy is required on the ward, saturations of 87-94% are acceptable in neonates, particularly premature neonates. Preoxygenation is still indicated prior to intubation, but if possible, avoid 100% FiO2 during the maintenance phase of anaesthesia.
Summary - practical implications for the anaesthetist:
- •Consider the normal values for respiratory rate by age
- •If a mechanical ventilator is available, select the appropriate tidal volume and respiratory rate for age – pressure control ventilation is preferred
- •Recognise the signs of respiratory distress; increased respiratory rate, grunting and recessions in an older child are extremely ominous
- •Neonates and infants have a high oxygen requirement and limited reserve; they become hypoxic rapidly if they are apnoeic or if the airway is obstructed. This is particularly important during induction of anaesthesia
- •Small children may not tolerate preoxygenation well, but you should always try
- •Gastric distension splints the diaphragm, the main muscle of respiration in neonates and infants; consider a nasogastric tube (NGT) in cases of gastric distension
- •CPAP/PEEP (positive end-expiratory pressure) are useful and may help avoid airway collapse and maintain oxygen saturations in neonates and infants
- •Select anaesthetic equipment with low deadspace volume to reduce the work of breathing Figure 3. Tracheal tug, subcostal and intercostal recession is common in babies with respiratory distress (illustration by Mrs P. Klebe, used with permission)
- •Monitor all babies for apnoeas after surgery; ex-premature babies are at increased risk until they are 60 weeks post conception
- •If oxygen therapy is required, SpO2 87-94% is recommended in premature neonates.
Cardiovascular considerations
Transitional circulation
With a newborn’s first breath, there is a transition from the fetal circulation (gas transfer at the placenta) to the newborn circulation (gas transfer at the lungs). Pulmonary vascular resistance decreases with the first breath by up to 80% (mainly due to the rise in PaO2 and in part due to the rise in pH and the fall in PaCO2 at birth). Systemic vascular resistance increases with clamping of the umbilical cord hence exclusion of the low resistance placental bed (see Figure 4).
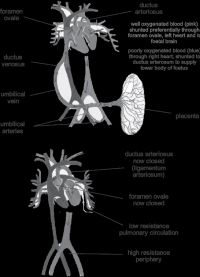
Venous return from the lungs to the left atrium increases and
the pressure gradient reverses across the foramen ovale, which
begins to close. The pressure in the pulmonary artery falls,
and blood flow through the ductus arteriosus is reversed so
that blood flows from the aorta to the pulmonary artery. The
ductus arteriosus begins to constrict due to increasing PaO2
and decreasing levels of prostaglandin E2 (PGE2). This is a
transitional period. The ductus does not undergo full fibrosis
for one month, and the foramen ovale may reopen in the first
5 years of life. Large decreases in systemic vascular resistance
or increases in pulmonary vascular resistance due to hypoxia,
hypercarbia, sepsis or acidosis in the first few weeks after birth
may cause the pulmonary vascular resistance to rise, and the
fetal shunts to reopen with right to left shunting; the baby will
become very cyanosed as deoxygenated blood flows from the
pulmonary artery to the aorta and pulmonary blood flow falls.
Worsening hypoxia leads to increased pulmonary vascular
resistance, which further amplifies the right to left shunt. This
is called persistent pulmonary hypertension of the newborn (PPHN).
Neonatal considerations
At birth the right ventricle is a similar size to the left ventricle, due to the high PVR in fetal life. There is therefore right-sided dominance on the newborn ECG. By two months of age the left ventricle is twice the size of the right, and a left dominant ECG is seen from 4 – 6 months of age. As the heart grows, the PR interval, QRS duration and the QRS size all increase. The newborn period is a time of rapid growth and development. High tissue oxygen delivery is required for the developing brain and other organs. The cardiac output is therefore relatively high compared to adults (see Table 4).
The ventricles are immature, and less compliant, with a relatively fixed stroke volume (1.5mls.kg-1 at birth), so increase in cardiac output is achieved through an increase in heart rate, rather than an increase in stroke volume as in adults (see table 3). This limits the ability to increase the cardiac output with a fluid challenge in a neonate, and it is easy to push the neonate into pulmonary oedema if too much fluid is given. Bradycardia (most commonly due to hypoxia) will reduce both cardiac output and blood pressure significantly.
In the newborn, vagal tone predominates. Hypoxia, airway manipulation, surgical stimuli and deep halothane anaesthesia are all likely to provoke bradycardia. Hypoxia should always be corrected and a dose of atropine (20mcg.kg-1) should always be drawn up when anaesthetising children. Start CPR if the HR drops below 60 bpm; small doses of adrenaline up to 10 mcg.kg-1 may be required if the heart rate is unresponsive to atropine.
Table 4. Changes in cardiac output with age
| Age of child | Cardiac Output (ml.kg-1.min-1) |
| Neonate | 300 |
| Adolescent | 100 |
| Adult | 70-80 |
Normal blood pressure varies with age, and can be estimated using the formula below (see table 3 for normal values):
Systolic BP (50th centile) = 85 + (age in years x 2)
Systolic BP (5th centile) = 65 + (age in years x 2)
As the child grows, the stroke volume rises, the heart rate falls and the systemic vascular resistance rises. The response to volume loading is more predictable from 2 years of age.
Summary – practical implications for the anaesthetist
- • Normal values for HR and BP vary with age
- • The response to a fluid challenge may be blunted in the neonate or small infant
- • It is important to avoid bradycardia. This should be treated rapidly should it occur; the most common cause is hypoxia
- • There is a ‘transitional’ circulation at birth, and fetal shunts may reopen in the critically ill neonate
Fluid balance
At birth total body water may be as high as 80%, which gradually decreases to 65% in the adult. Extracellular fluid accounts for 40% of this volume (higher in prematurity), more than in adult patients. Children are particularly prone to dehydration as they have a higher metabolic rate and extracellular fluid turnover, they do not concentrate the urine well to conserve water, and infants also have a higher surface area to weight ratio with relatively higher insensible losses. There is poor compensation for extracellular fluid dehydration as the intracellular compartment is relatively small. The stress response to surgery may result in hyponatraemia due to the release of antidiuretic hormone (ADH) from the pituitary, over-riding the effect of plasma osmolarity (see chapter on fluids, page 81). The use of hypotonic fluids (such as 0.18% saline with 4% glucose) may exacerbate this and should be avoided. Normal saline or Ringers lactate would normally be chosen for fluid maintenance. Due to the risk of hypoglycaemia, glucose containing solutions should be given to neonates or children below the 3rd centile of weight (for example 0.9% saline with 5% dextrose). Use close monitoring of sodium and glucose levels when giving IV fluids in these patients.
It is important to monitor and replace intraoperative losses
meticulously. It is estimated that 10mls.kg.-1hr.-1 evaporative
losses occur during paediatric laparotomy. Swabs should be
weighed and suction fluid losses measured carefully.
Summary – practical implications for the anaesthetist:
- • Children are prone to dehydration, and should not be fasted excessively. Allow free clear fluids (water) up to 2 hours before surgery
- • Fluid balanceshould be monitored carefully during surgery
- • Children are prone to hyponatraemia in the perioperative period; use only isotonic fluids.
Haematology
At birth 70% of haemoglobin is HbF; this has alpha and gamma haemoglobin chains, and is ideally suited to efficient delivery of oxygen to the tissues in the hypoxic environment in the fetus. However, tissue oxygen delivery by HbF at the higher values of PaO2 found in the newborn is less efficient. Haemoglobin concentrations, blood volume and cardiac output are relatively high in the newborn to facilitate oxygen delivery to the tissues, and to meet the high metabolic demand for oxygen. Premature neonates may have a low haemoglobin as iron stores are laid down in the final three months of gestation. HbF declines to negligible levels by 6 months of age. Production of haemoglobin HbA2 increases from birth, and reaches adult levels by 6 months of age; ‘physiological anaemia of infancy’ is seen at 3-6 months as HbF levels decline and HbA2 levels increase (see Table 5 for normal values). The relative blood volume in neonates is high, but the absolute volumes are very small. If the absolute volume is 300mls, blood loss of just 60ml is 20% of the circulating volume. Transfuse blood (packed red blood cells) if 20% of blood volume is lost or if the haematocrit falls to less than 25%. Consider clotting factors, platelets and fibrinogen (or use whole blood) if blood losses are more significant. (See the article on ‘major haemorrhage’ for more information, page 195).
Thermoregulation
Heat stores in children are small due to their low body mass. Heat is lost rapidly due to high evaporative losses, and poor insulation:
Table 5. Haemoglobin levels and blood volume – normal values
| Age group | Blood volume (mls.kg-1) | Haemoglobin concentration (g.l-1) |
| Neonate | 90 | 180-200 |
| Infant | 85 | 90-120 |
| Child | 80 | 110 |
| Adult | 70 | 110 |
- • Large surface area to body weight ratio
- • High minute ventilation
- • Thin skin
- • Poor subcutaneous fat stores.
Neonates are unable to maintain body heat as the thermoregulatory centres are immature, and they cannot shiver or sweat efficiently. They can generate heat through the metabolism of brown fat (non-shivering thermogensis). Brown fat accounts for about 5% of a neonate’s body weight and is distributed around the scapulae, kidneys, adrenals and mediastinum. Metabolism is neurally mediated (ß3 adrenoreceptors) and generates heat. This is inefficient and increases oxygen consumption. Older children (>3 months) have ineffective shivering due to limited muscle mass. Vasconstriction in children is inefficient.
Children are therefore prone to hypothermia. The consequences of hypothermia include:
- • Increased oxygen consumption
- • Respiratory depression
- • Cardiac arrythymias
- • Coagulopathy
- • Acidosis
- • Hyperglycaemia
- • Immunosupression
- • Prolonged drug metabolism.
Keeping children warm improves outcomes. (See Equipment article for strategies, page 13).
Renal considerations
Renal vascular resistance is higher in the newborn and there is a relatively low renal blood flow and glomerular filtration rate. The ability to concentrate urine is poorly developed as the renal cortical tubules are immature; babies therefore produce large volumes of dilute urine and do not tolerate dehydration well. Sodium is required for normal growth, and sodium is actively conserved. Dietary sodium requirements may be higher in premature neonates due to increased renal sodium losses. Expansion of the plasma fluid volume should be avoided in the first few days of life before the post-natal diuresis has occurred; babies do not require added sodium if they receive IV maintenance fluids in the first few days of life; add sodium should be added to maintenance fluids after the first few days of life. Isotonic fluid should always be used during surgery. Urine output in the newborn is approximately 1-2mls.kg-1.hr-1.
Hepatobiliary considerations
Neonates have poor liver glycogen stores and are at risk of hypoglycaemia. A 10% dextrose infusion may be required to prevent this. There is an increase in red cell breakdown and a limited ability to handle unconjugated bilirubin, so physiological jaundice is common in the first two weeks of life. Vitamin K dependent clotting factors (II, VII, IX and X) are deficient in the newborn and ideally, vitamin K should be given at birth to prevent haemorrhagic disease of the newborn, particularly in a neonate who requires surgery. Platelet function is reduced and there is a risk of bleeding, particularly in the septic neonate (the platelet count falls in sepsis). Hepatic metabolism of drugs is reduced in the neonatal period, and may take 3 months to reach full activity. This is particularly relevant to opioids - the half-life of morphine is 6-8 hours in the term neonate compared to 2 hours in infants and older children, so increased dosing intervals should be used and opioids strictly titrated to effect in neonates.
Immune system
The neonate is relatively immunosuppressed. Maternal IgG antibodies, which can cross the placenta, fall during the first 6 months. Breast-feeding helps protect against gastrointestinal and respiratory tract infections.
Nervous system
The central nervous system is immature at birth and cerebral myelination continues for up to 3 years. The process of
Table 6. Central nervous system – normal values
| Patient group | Cerebral blood flow (ml.100g-1.min-1) | Cerebral metabolic rate for oxygen (CMRO2) (ml.100g-1.min-1) | CSF volume (ml.kg-1) | CSF volume (ml.kg-1) |
| Newborn | 50 | 4 | <2 | |
| Child | 100 | 5.8 | ||
| Adult | 50 | 3.5 | 2 | 7-15 |
Table 7. Comparison of volatile agents in children
| Halothane | Sevoflurane | Isoflurane |
| MAC (%)
Infant: 0.9 Child: 1.2 Adult: 0.75 |
MAC (%)
Infant: 3.3 Child: 2.5 Adult: 1.7 |
MAC (%)
Infant: 1.6 Child: 1.9 Adult: 1.2 |
| Sweet smelling, well-tolerated | Non-irritant | Pungent, irritant, difficult to use for
inhalational induction |
| High lipid solubility, slow onset/offset anaesthesia; induction rate increased if used with nitrous oxide. | Low lipid solubility, rapid onset/offset of anaesthesia | Low lipid solubility, rapid onset/offset of anaesthesia |
| Arrhythmias common, myocardial depression common | Good haemodynamic stability; blood pressure may fall due to fall in systemic vascular resistance | Good haemodynamic stability; blood pressure may fall due to fall in systemic vascular resistance |
| Respiratory depression seen | Respiratory despression common | Respiratory depression seen |
| Inexpensive | Expensive, although costs coming down | Expensive, although costs coming down |
| Hepatic metabolism; halothane hepatitis seen rarely (repeat anaesthetics) | Not highly metabolised | Low hepatic metabolism |
| Compatible with draw-over systems; not all modern anaesthesia systems include a halothane vaporiser | Compatible with modern anaesthesia systems; not all draw-over systems include a sevoflurane vaporiser | Compatible with modern anaesthesia systems; can be used in a halothane vaporiser |
central nervous system maturation continues during fetal
and neonatal development, but the ability to feel pain is well
developed even before birth. Pain in neonates is associated
with behavioural changes such as tachycardia, hypertension,
grimacing, crying and restlessness; pain scores in children
take these behavioural features into account. There is concern
about the effect of anaesthetics on the developing brain, and
unnecessary anaesthetics should be avoided in infancy.
Cerebral blood flow is high in childhood, and intracranial
pressure (ICP) is lower in the newborn (see table 6 for normal
values). Rises in intracranial pressure are well-compensated
in infancy, in part by expansion of sutures and bulging of
fontanelles, and intracranial pressure can be estimated by
palpation of the fontanelles. Ossification of the fontanelles is
complete by about 18 months.
Pharmacological considerations
Volatile anaesthetics
Neonates are more sensitive to volatile agents than older children, and the minimum alveolar concentration (MAC) values are decreased in neonates but increased by up to 30% in infants and children compared to adults. Halothane and sevoflurane remain the agents most popular for inhalational induction of anaesthesia (see table 7 for comparison between volatile agents).
Sedatives and hypnotics
Children are particularly sensitive to sedative and hypnotic drugs such as barbiturates and benzodiazepines due to the immature blood brain barrier and reduced drug metabolism/ excretion; these drugs should be used with caution, in weight Update in Anaesthesia | www.wfsahq.org/resources/update-in-anaesthesia page 11 appropriate doses, titrated according to effect. Propofol infusions may be used in children older than 3 years. See article ‘Paediatric procedural sedation’ on page 77).
Muscle relaxants
The volume of distribution of water soluble drugs is higher at birth due to the increased extracellular fluid volume. (This is true for all water soluble drugs, not only muscle relaxants). This explains the need for a higher dose of suxamethonium in infants relative to adults (2mg.kg-1 vs 1mg.kg-1). A second dose of suxamethonium may provoke bradycardia due to the high parasympathetic tone in infants; atropine should always be available.
Neonates and infants are more sensitive to non-depolarizing neuromuscular blocking drugs. A normal loading dose is given but subsequent doses should be reduced.
Analgesics and local anaesthetics
Drug doses need to be reduced due to immature metabolism, and in the case of local anaesthetics, lower levels of plasma binding proteins (serum albumin and alpha-1 acid glycoprotein). Take extra care not to exceed maximum doses or dose intervals of analgesics such as paracetamol, ibuprofen, opioids or local anaesthetics. (This topic is considered in more detail on page 72).
Acknowledgements
(included from the original edition)
I would like to thank Dr Steven Froom, Consultant Paediatric Anaesthetist for a review of the final draft and my sister, Mrs Penelope Klebe, for her excellent illustrations.
Further reading
1. Berg S. Chapter 33: Neonatal/infant physiology. In: Allman KG, Wilson IH, eds. Oxford handbook of Anaesthesia (3rd edition) Oxford: Oxford University Press, 2011: 800-809.
2. Jewitt H. Chapter 2.8: Principles of Neonatal Physiology. In: Allman KG, Wilson IH, eds. Dr Podcast Scripts for the final FRCA (1st edition) Cambridge: Cambridge University Press, 2011: 325-331.
3. Macfarlane F. Paediatric anatomy and physiology and the basics of paediatric anaesthesia. World Federation of Societies of Anaesthesiologists 2005; Tutorial of the week number 7.
4. Brown K. The application of basic science to practical paediatric anaesthesia. Update in Anaesthesia 2000; 11: 73-77.